COA Reports

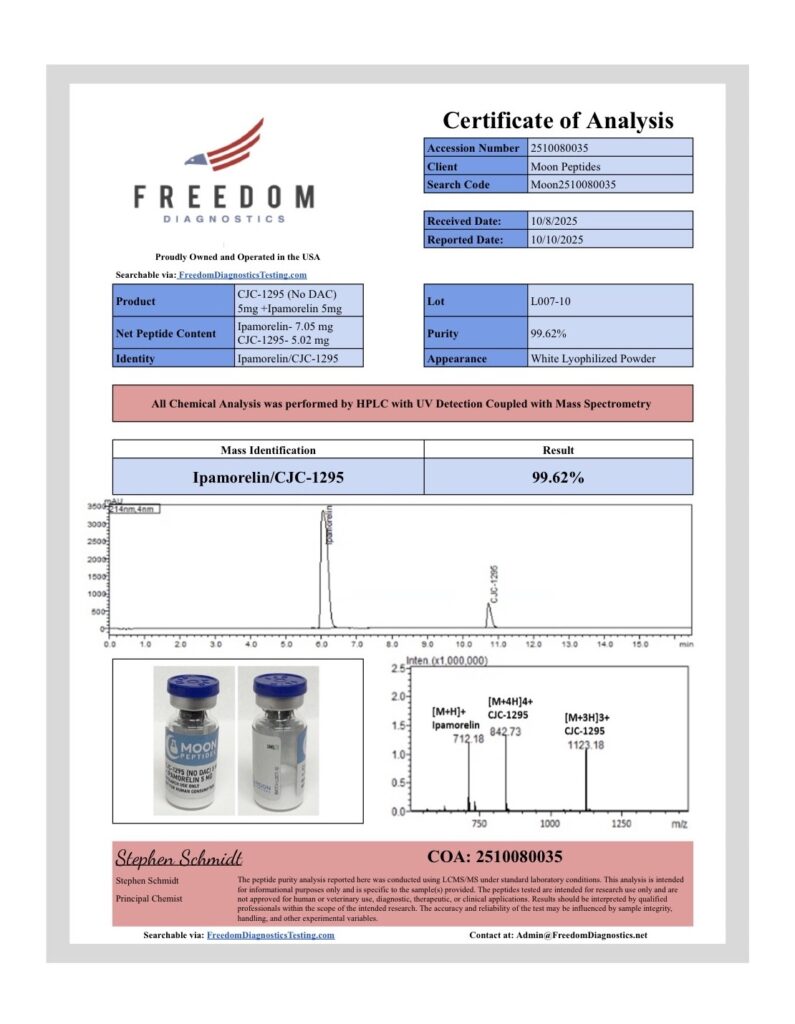
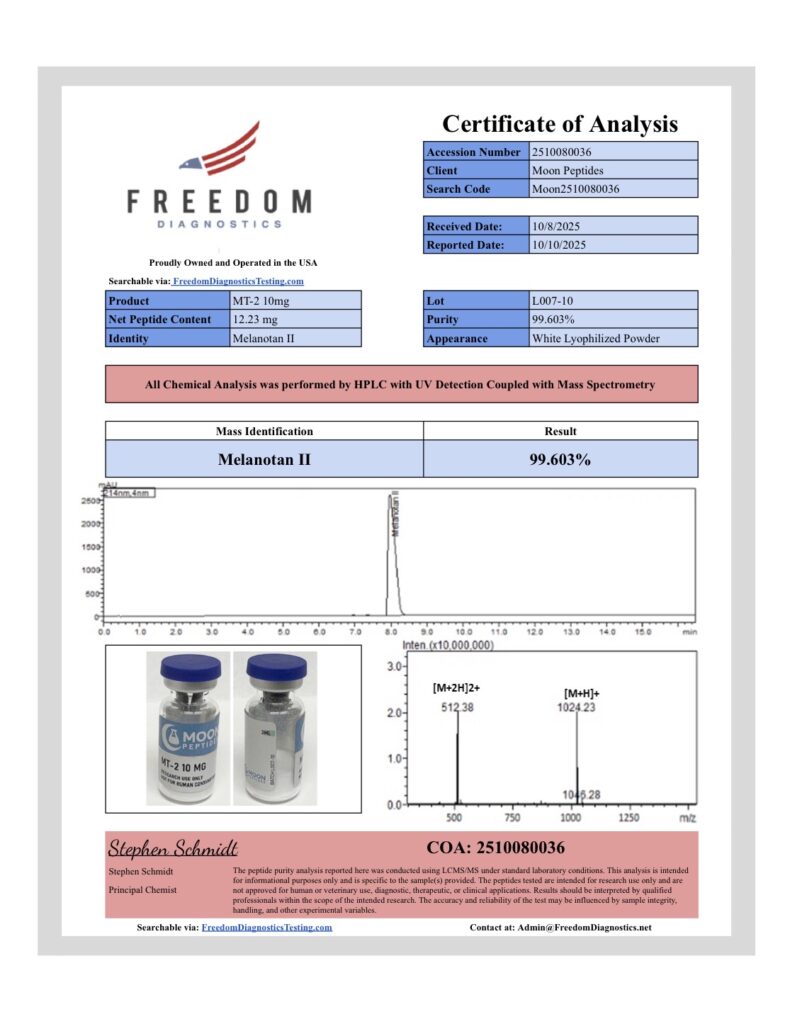
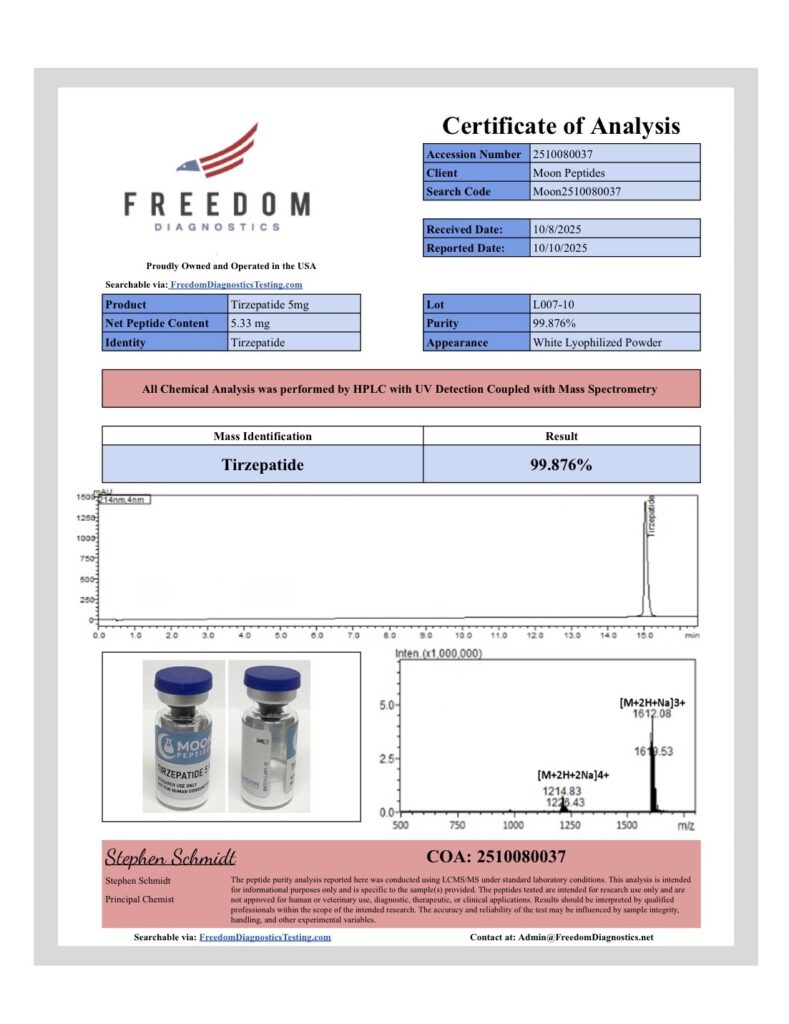
At Moon Peptides, quality verification is handled through independent third party laboratory testing to help confirm identity and assess purity. Each batch is tested using analytical methods such as HPLC and Mass Spectrometry, with results documented on the Certificate of Analysis for that specific lot.
Purity results of 99 percent or greater are reported where confirmed by the third party lab and reflected on the corresponding COA. Our batches are produced using controlled manufacturing and quality practices aligned with GMP principles, with lot traceability and documentation maintained for review.
Certificates of Analysis
We routinely update this page as new batches arrive and new COAs become available. Each COA typically includes key batch details such as the lot number, test date, analytical method, and reported results, so researchers can properly document materials used in their work.
Request a COA
If you would like a copy of a specific product’s COA, email Sales@moonpeptides.com
with:
Product name
Order number
Lot number, if available
We will provide the verified documentation for the applicable batch.
Disclaimer:
All products are for laboratory research use only. Not for human consumption.